The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.
Anthony Grilli is owner of FOCUS Scientific Services, a cGMP consulting firm. He has more than 20 years of analytical laboratory management experience and has managed several extractable and leachable projects for drug manufacturers.
Biopharmaceutical packaging performs several vital functions in assuring the drug product safety. First and foremost, packaging must be a barrier to the external environment and maintain the sterility of its contents. Depending upon the contents, packaging may also serve to shield the drug product from oxidation, light degradation, and moisture permeation. The packaging must clearly identify its contents and may include dosage information and hazard warnings. Finally, a package may help to ensure accurate dosage of a drug product, in an easy and foolproof way. Satisfying all of these functional requirements includes testing of a spectrum of components and materials: plastic containers, metal springs, elastomeric valves and gaskets, adhesives, and coatings. While these materials may meet the functional goals of the container closure, if quality is considered at the beginning of the process, harmful contaminants may inadvertently be introduced into the drug product.This is not just a thought experiment, as there is ample history of harmful packaging additives leaching to stored contents. Up until the 1980s, carbon black was added to rubber to make it more supple. It was also added to elastomers used in everything from asthma inhalers to baby bottle nipples, until it was shown that cancer causing polyaromatic hydrocarbons leached from rubber made with carbon black (1). Bisphenol A is used as a building block in polycarbonate bottles and as a liner in metal cans, and was common in baby bottles, but is now known to be an endocrine disruptor and is a banned plastic additive in several states.
Complexity of packaging means that a wide range of materials are involved. Therefore, potentially harmful leachables, including (but not limited to) metals, catalysts, antioxidants, curing agents, activators, accelerators, pigments, stabilizers, plasticizers, and lubricants can be introduced. How does one set up a testing program to ensure none of these potential components are contaminating the final product? Is it reasonable to test for every potential impurity?
A standard extractable and leachable program begins by coaxing potential leachables from packaging or processing materials in an exaggerated extraction study. Components are shredded and placed in solvents of varying polarity, regardless of final drug product solvent. This solvent component mixture is heated at elevated temperatures to extract all potential impurities out in a short period of time. These extracted chemicals are then identified by various analytical techniques, typically inductively coupled plasma mass spectrometry (ICP– MS), liquid chromatography–mass spectrometry ( HPLC– MS), and gas chromatography–mass spectrometry (GC–MS). Extractables of concern are highlighted and targeted in the leachable study. Not all extractables are leachables, but because it is not always clear which components could leach out under storage conditions likely to be encountered, methods are developed to detect the extractables in the product matrix. During finished product testing, it will be useful to quantify the leachable impurity in the presence of the drug product. These impurity methods are validated for accuracy, precision, specificity, linearity, range, and limit of quantitation. These validated impurity tests are then added to the suite of tests run during the product stability study. Extracting, identifying, developing, and validating methods for these components is a costly and labor-intensive endeavor and resembles a “quality-byQC test” strategy, rather than a more sensible quality by-design (QbD) strategy that is expected today. If harmful leachables are found during stability studies, one would have to step backwards toward product design stages, choose new materials, and qualify them.
FDA Guidance for Industry, Q8 (R2) Pharmaceutical Development describes the elements of QbD used in pharmaceutical development (2). The guidance outlines a series of proactive steps used to build quality into the final product. Figure 1 illustrates these steps.
Figure 1: Developing a container/closure system utilizing quality by design (QbD) to minimize the impact of leachables.
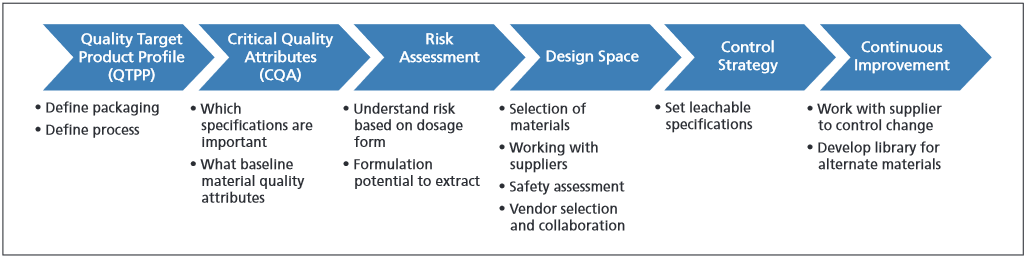
The first QbD element is quality target product profile (QTPP). The QTPP is the basis for the design of the drug product and its packaging. It is at this stage that the team will describe intended use, route of administration, dosage form, patient population, chronicversus short-term use, etc. Any changes in the QTPP will require evaluation of the imp ct of this change in proceeding uality design elements. QTPP information informs the second element of QbD, the critical quality attributes (CQA).
CQA is defined as a physical, biological, or microbiological property or characteristic that should be within an acceptable range to ensure the desired product quality. The FDA guideline states that CQAs are generally associated with drug substances, excipients, intermediates, and drug product, but the concept can also be applied to container closure systems. While it is not possible at the QTTP stage to define all potentially harmful leachables, one can certainly prohibit known harmful additives such as mercaptothiazole or bisphenol A. One can also set baseline CQA that require packaging and production components meet basic entry-level quality attributes. Examples include the gravimetric leachable criteria for nonvolatile residues, residue on ignition, heavy metals, and buffering capacity outlined in United States Pharmacopeia (USP) <671> Containers—Plastic (3). Glass composed should minimally meet impurity resistance and etching teral product criteria outlined in USP 37 <660> Containers—Glass (3). Gross toxicity screening should also be confirmed for plastic and elastomeric components, such as that provided by USP <88> Biological Evaluation Reactivity Tests, In Vivo (3), confirming the plastic meets USP Class VI criteria. Impact on cell culture might also be important, particularly for single-use fermentation bags, for example, and USP <87> Biological Evaluation, In Vitro (3) would be a reasonable minimal CQA for which to start.CQAs for specific leachables are added after conducting extractable risk assessment. The relative risk to patient health of design parameters outlined during the QTPP is evaluated and informs CQAs. Consider, for example, the analysis of risk poses by doage form. A leached in an inhaliation or patrent leading poses a higer risk than able and leachables the same impurity in an oral or topical product. Similarly, aqueous drug products pose a higher likelihood of drug product interactions than solid oral dosage forms. Therefore, a broader range of leachables at lower detection levels is suggested by liquid parenteral or solvent propelled inhalant drug packaging. Table I and Table II from FDA Guidance for Industry, Container Closure Systems for Packaging Human Drugs and Biologics (4) illustrate this relationship.
USP <660> and <661> (3) are baseline tests that should be added to all container closures. As risk of exposure increases, so does complexity of testing.
Table I: Route of administration and potential harm from leachables, compiled from FDA Guidance for Industry, Container Closure Systems for Packaging Human Drugs and Biologics.
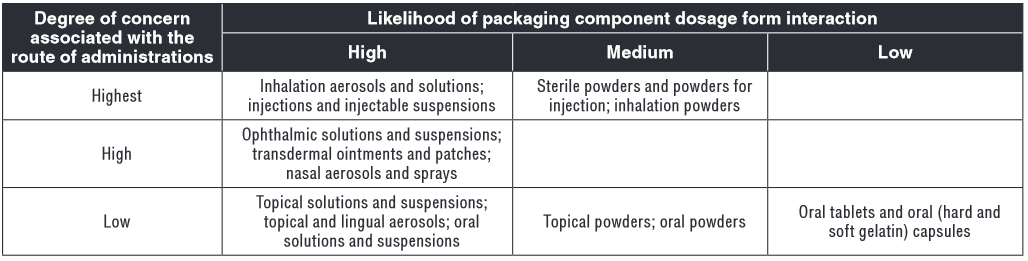
Table II: Route of administration and tests to assess safety of potential leachables, compiled from FDA Guidance for Industry, Container Closure Systems for Packaging Human Drugs and Biologics.
An Ishikawa diagram (Figure 2) can be made by the design and quality team to asses potential risks onents to a reasonable extract hydrolytic assessment.
Figure II: Ishikawa diagram to assess leachable risk associated with design variables.
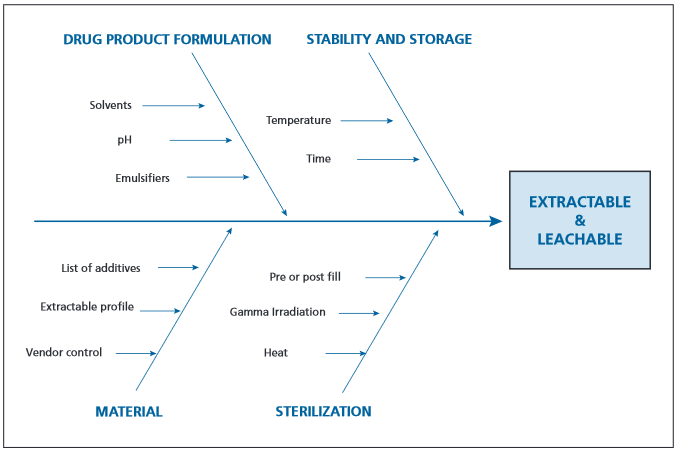
Consideration should be given to materials sourced, formulation, storage conditions, and sterilization techniques.
An example of using risk analysis to inform CQA is in the Product Quality Research Institute’s establishment of the analytical evaluation threshold (AET) for inhaled drugs. The AET is the threshold at or above which one should identify a particular extractant and make a toxicological assessment. The calculation for AET relies on a safety concern threshold (SCT) of 0.15 mcg/day. The SCT is the threshold below which a leachable would have a dose so low as to present negligible safety concerns from carcinogenic and non-carcinogenic effects (5). If the potential exposure of the patient exceeds 0.15 mcg/day, the extractant should be identified and the toxicity should be assessed. Anything below this value is considered low risk.
The relationship between the materials selected, the manufacturing process, and critical quality attributes are described in the design space. This defined relationship will provide assurance of quality in the final product. Extractable studies have been performed, risk analysis conducted, and CQAs are established. Within the design space, it is important to work with the packaging component supplier to understand what potential sources of variability exist for each packaging component, especially those with greatest product contact and highest risk. Confirmation that the sup-plier has cGMP control over its manufacturing process is essential. Once variability is known, one can assess the impact of this variability on component leachables, and in turn, how these leachables may affect drug product quality.
Jenke and Swanson describe the use of a design space in the extractable and leachable assessment of terminally sterilized aqueous drug product in a plastic packaging system (6). Quality variables controlled in the design space included composition of drug product; composition of packaging system; configuration of packaging system; and conditions of contact. When controlling for these variables, the product package interaction and leachable parameters within the design space can be so well known after sufficient evaluation, that subsequent leachable profiles can be predicted.
The next step in the QbD process is the control strategy. What are the planned sets of controls, based on the design space that will ensure end-product quality? It is sensible to apply control upstream— to the component extractable specification—where the source of variability is likely. If the component has a new extractable due to lack of manufacturing consistency, it may not be picked up during leachable assessment if the wrong column or method is used. A supplier’s pharmaceutical application for a component or resin may be a small piece of its overall business profile, particularly for commodity-type components like metal springs or plastic pieces. The importance of change control and supply chain management for these vendors cannot be overemphasized.
Finally, there is life cycle management and continual improvement. It is important to partner with the material supplier to understand potential changes in their processes. A vendor quality agreement will help ensure adequate notification of any changes in process or materials. It may be useful to proactively source and assess alternative materials, in case of business failure or unacceptable change. Change of the design space occur after gaining addition l process knowledge.
QbD inciples allow for a more useful extractable and leachable program. Rather than simply focusing on identification of all potential leachables in the finished product, QbD takes a more holist approach to ensure product quality and focuses on the leachables that have an actual risk to product integrity. QbD also considers potential product quality influencers outside of the testing program, such as issues with vendor qualification and disruptions in supply chain management.