Acknowledgment
This study was conducted at an independent laboratory, supported by funding from QI Medical.
Abstract
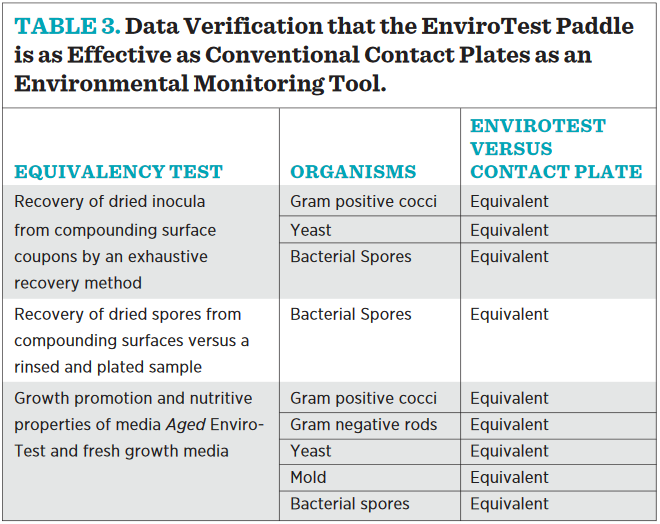
This article provides the results of a study evaluating the sampling efficiency of a flat paddle sampling device against a round surface sampling device in controlled studies. each device is supplied with identical microbial-growth media, and laboratory studies document equivalent performance of the media in the devices. both devices perform sampling by the same process (agar transfer of microorganisms from the facility surface to the agar surface), and the performance of the two devices was shown to be equivalent in laboratory studies. either the commonly used flat paddle sampler or the round contact plate is suitable for surface monitoring in the compounding pharmacy.
The Purpose of Environmental Sampling
The purpose of environmental sampling in the compounding pharmacy is to monitor the state of control of the cleanroom environment. As we are most concerned with microbial contamination, microbial monitoring is the most direct measure of this control. However, microbial monitoring is inherently variable, and a single-day’s samples may be subject to enormous variability.1-5 This variability requires frequent sampling and trending of the data to identify when the facility is beginning to drift out of control.
The most important consideration in determining how to design and execute an environmental monitoring program is to maintain its purpose clearly in mind. This purpose is to monitor the state of microbial control of the compounding area. The U.S. Food and Drug Administration (FDA) made its expectations clear in the 2004 Aseptic Processing Guidance document in section X.A.1, where it is stated6:
In aseptic processing, one of the most important laboratory controls is the environmental monitoring program. This program provides meaningful information on the quality of the aseptic processing environment (e.g., when a given batch is being manufactured) as well as environmental trends of ancillary clean areas. Environmental monitoring should promptly identify potential routes of contamination, allowing for implementation of corrections before product contamination occurs (211.42 and 211.113).
The issue of sufficient environmental monitoring is one of the most frequently cited topics in FDA inspections of the compounding pharmacy.7-9
An effective environmental monitoring program for a compounding pharmacy producing compounded sterile preparations (CSPs) should therefore involve regular sampling of both air and surfaces to permit this data trending.10 Recently released draft guidance for 503B Outsourcing Facilities11 requires daily monitoring, although FDA’s draft guidance on 503A facilities is silent on this point.12
United States Pharmacopeia (USP) <797> is not in complete agreement with the 503B expectations, stating13:
Environmental sampling shall occur as part of a comprehensive quality management program and shall occur minimally under any of the following conditions:
- as part of the commissioning and certification of new facilities and equipment;
- following any servicing of facilities and equipment;
- as part of the re-certification of facilities and equipment (i.e., every 6 months);
- in response to identified problems with end products or staff technique;
- or in response to issues with CSPs, observed compounding personnel work practices, or patient-related infections (where the CSP is being considered as a potential source of the infection).
USP Chapter <797> then goes on to reference USP Chapter <1116> for further information. Chapter <1116> is at odds with the bulleted list above, as are the FDA expectations, both of which expect a frequent sampling plan and trending of data. Hopefully, this discrepancy will be resolved soon.
However, it is clear that whether a 503B or 503A compounding pharmacy, the modern compounding pharmacy is expected to have a suitable environmental-sampling program in place.
Given the importance of this question, the current study was undertaken to specifically compare the suitability of different devices for use in surface sampling in the compounding pharmacy.
The Sampling Devices
The sampling devices most commonly used are contact plates and flat paddle samplers, both of which rely on agar-growth media projecting above the device. The agar is pressed onto the surface to be sampled and microorganisms adhering to the agar surface are captured for subsequent growth and analysis. This process can be thought of under the general mechanism of “agar transfer” of organisms from the flat surface. USP <797> does not mandate the use of a specific device, stating only that “The size of the plate to be used for each sampled location usually ranges [italics added for emphasis] from 24 to 30 cm2.”13
The flat paddle sampling devices have been in use for many years for a variety of purposes.14-17 Their use in pharmaceutical cleanrooms is well established,6,18 as is the use of sampling paddles and contact plates in compounding pharmacies.19-21 In fact, recent
surveys show they are currently in widespread use in the pharmacy environment.19,20
Flat paddle sampling devices have been shown to be equivalent in efficacy to the round contact plates. In a pair of studies by Salo’s group at the VTT Technical Research Centre of Finland,22,23 the efficacy of the rectangular flat paddles, circular contact plates, and swabbing methodology was studied.
The first study22 was a collaborative effort among 12 sites evaluating total aerobic microbial recovery using Soybean Casein Digest Agar as the recovery medium (see discussion of media below). This study compared the Hygicult TPC dipslide (a flat paddle sampler marketed by Orion Diagnostica) against contact plates and swabbing. The challenge used stainless steel surfaces artificially contaminated with different microbes at various levels. The Hygicult TPC dipslide, contact plate, and swabbing methods gave similar results.22
The second study23 was a similar collaborative study, but in this case with Enterobacteriaceae to compare Hygicult E dipslides with violet red bile glucose agar (VRBGA) contact plates and swabbing. They used stainless steel surfaces to determine recovery of enteric bacteria. The results of this sudy were the same as in the Salo 2000 study—no significant differences were seen in the recovery of the challenge organism between the sampling methods. Finally, the flat paddle devices are by far the most commonly used surface sampling devices in compounding pharmacies over the past years, as determined by independent surveys.19,20
In this study, we looked at more detailed methods of comparison between the different devices and at the effect of aging on the nutritive capabilities of the media. It is important to remember that the different devices use identical media, and so could reasonably be expected to perform similarly in microbial growth promotion (nutritive) capabilities and in sampling efficiency. However, there is little recent data to support this assumption. This study looks in detail at these questions.
Materials and Methods
A pair of controlled studies was performed at a Current Good Manufacturing Practices (cGMP) laboratory with the purpose of:
- Study #1: Comparing each device’s (EnviroTest and contact plate) ability to lift and grow dried microbes from typical compounding surfaces. This test is based on designs described in USP <1072>24 to determine sampling efficiency of surface sampling methods; and,
- Study #2: Evaluating the nutritive properties of each device’s media component. This study was designed to stress the ability of the EnviroTest screw-cap package to maintain the nutritive agar by comparing aged EnviroTest to fresh control media. This study was designed on well-established methods described in USP <71>,25 USP <61>,26 and by Weenk.27
Testing Summary
Test Species
- Bacillus subtilis ATCC 6633 NCTC 10400
- Staphylococcus aureus ATCC 6538 NCTC 10788
- Pseudomonas aeruginosa ATCC 9027 NCTC 12924
- Candida albicans ATCC 10231 NCTC 3179
- Aspergillus brasiliensis ATCC 16404 NCTC 2275
These challenge organisms were chosen as well-controlled index organisms, commonly used in USP testing for different quality tests. Test species were prepared from BioBall inoculation products, a precise quantitative inoculum patented and manufactured by Biomerieux. The BioBall product uses a flow cytometer to dispense individual cells and count them in each inoculum, resulting in a very precise number of cells.
Devices
- Envirotest Tryptic Soy Agar Paddles: Contains tryptose, yeast extract, dextrose, agar, lecithin, and polysorbate 80 (Lots 1556804, 1564724, 15790764)
- Envirotest Malt Extract Agar Paddles: Contains malt agar, yeast extract, dextrose, lactic acid, antibiotics, agar, lecithin, polysorbate 80, histidine, and thiosulfate (Lot 1562027)
- Emd Millipore Tryptic Soy Agar Contact Plates: Contains tryptose, yeast extract, dextrose, agar, lecithin, and polysorbate 80 (Lots 123051, 122852, 125136)
- EMD Millipore Malt Extract Agar Contact Plates: Contains maltose, dextrin, glycerol, peptone, agar, lecithin, and polysorbate 80 (Lot 1331518)
These devices are widely employed for surface monitoring in the industry. Each is commonly available, filled with the recognized microbial growth media Trypticase (Tryptic) Soy Agar28 and Malt Extract Agar29. Commercially sourced sampling devices were used in this study.
Procedure For Study #1: Microbial Recovery From Facility Surfaces
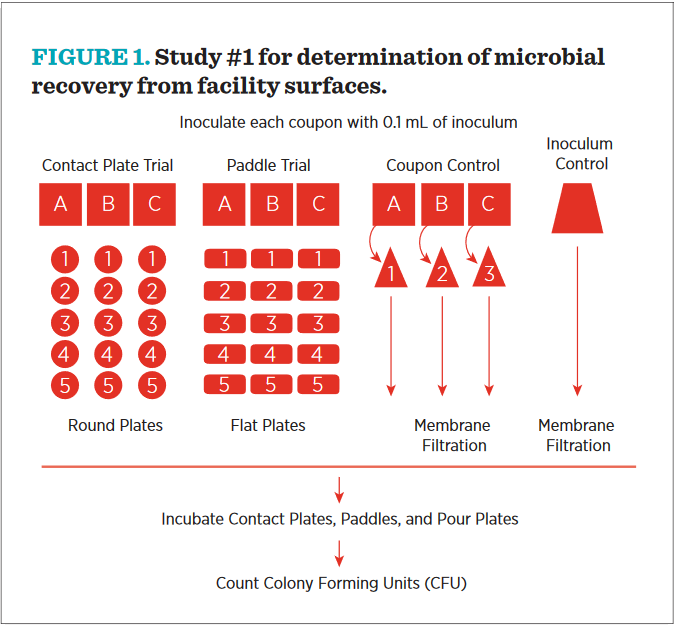
A diagrammatic overview of Study #1 is shown in Figure #1. Note that there are four populations for comparison—coupons (denoted “A,” “B,” and “C”) sampled repeatedly with round plates, coupons sampled with the EnviroTest flat paddle, three coupons suspended in liquid for recovery, and an inoculum control.
- Coupon preparation
- Surfaces used in Study #1:
- Vinyl tile flooring material
- Borosilicate glass
- Stainless steel
- Materials were cut to 2 × 2 inch coupons.
- The steel and glass coupons were cleaned by rinsing with deionized (DI) water and sterilized in an autoclave.
- The vinyl coupons were cleaned by rinsing with DI water and sterilized in an ethylene oxide sterilization cycle. The coupons were allowed to aerate for 4 days prior to testing.
- Microbial inoculation
- BioBall culture preparations were removed from freezer and allowed to equilibrate at room temperature prior to use.
- BioBall was transferred into the rehydration fluid, the cap was replaced, and the inoculum dissolved for 30 seconds.
- The resulting mixture was vortexed for 5 seconds or until Bioball was completely dissolved and there was a visibly homogenous distribution.
- Nine replicates of each coupon type were placed in 9 individual petri plates. Three plates were labelled for Contact, 3 were labelled for EnviroTest, and 3 were labelled for Control.
- Each of the replicate coupons was inoculated with 0.1 mL of 1.0 × 103 colony forming units (CfU) of prepared microbial suspension. The inoculum was spread evenly across the coupon, leaving approximately 1/4 inch on the left and right side of the coupon free of inocula.
- A 10-mL phosphate buffered saline blank was simultaneously inoculated with 0.1 mL of 1.0 × 103 CfU.
- Inoculated coupons were dried in a 35°C to 39°C incubator with petri plate lids on. Inoculum was allowed to dry completely.
- Enumeration
- Contact Plates: Surface of coupon was pressed with a contact plate, firmly enough so that the entire surface of the agar was in contact with the coupon. Plate was held in this position for 5 seconds. Contact plate was removed and lid was replaced. Each tile was sampled an additional four times, with four fresh contact plates, so that an initial recovery percentage could be calculated from the five sequential samplings.
- EnviroTest: Surface of coupon was pressed with a paddle, and the paddle was gently rocked. The paddle was then flipped and the other side was pressed to the other half of the coupon, again slightly rocking the paddle. The paddle was removed from coupon and placed back in the tube. Each tile was sampled an additional four times, with four fresh paddles, so that an initial recovery percentage could be calculated from the five sequential samplings.
- Control Tile: Inoculated coupon was placed in whirl pack bag with 10 mL phosphate buffered saline. The coupon was thoroughly massaged through the bag to dislodge all inoculum from coupon. The resulting phosphate buffered saline was passed through a 0.45-micron filter. filter was placed on TSA+ for bacteria and MEA+ for fungi. This sample was assumed to represent 100% recovery of all surviving microorganisms on the tiles and controlled for loss of microorganisms by dessication.30
- Bacterial plates were incubated at 30°C to 35°C for 3 days, fungal plates were incubated at 20°C to 25°C for 5 days, and CfUs were counted.
- Calculations
- The “Percent Recovery” was determined by a variation of the “Recovery to Exhaustion” method where the initial number of CfU recovered from the coupon is used to estimate recovery efficiency by comparison to the total number of CfU recovered from multiple samplings of the same surface. This was expressed as

- The comparative recovery of a device for a specific material was determined by comparison to an untreated control tile. This was expressed as:

Procedure For Study #2: Comparing Nutritive Quality Of Two Devices
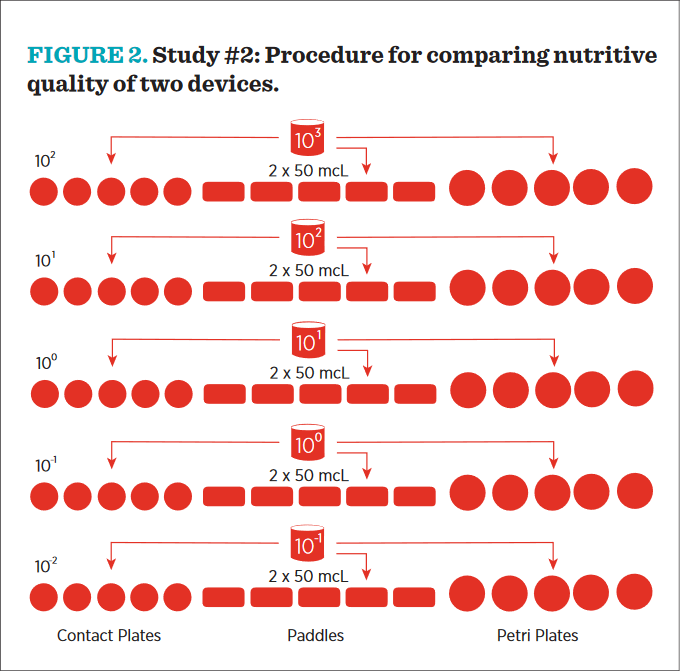
The basic design for this study was suggested by Weenk, who noted the utility of the “Most Probable Number” (MPN) in growth promotion style studies.27 This method is a statistical comparison of replicate sample dilutions expected to contain very low numbers of microorganisms and, for this purpose, is more accurate than plate-count estimates. By comparing derived MPN values from comparative media inoculated with identical microbial dilutions, any variation between the derived MPN must be due to the nutritive capabilities of the media being compared. The procedure followed in this study is diagrammed in Figure 2 and described below:
- Control media used in study #2:
- Tryptic Soy Agar Plus Neutralizers (TSA+) Control Plates: Contains tryptose, yeast extract, dextrose, agar, lecithin, and polysorbate 80. Prepared by the testing laboratory from a commercial blend.
- Malt Extract Agar Plus Neutralizers (MEA +) Control Plates: Contains maltose, dextrin, glycerol, peptone, agar, lecithin, and polysorbate 80. Prepared by the testing laboratory from a commercial blend.
- Five replicates devices (contact plates and paddles) were labelled for each organism and each dilution: 101, 100, 10-1, 10-2, and 10-3. The media used for these replicates were TSA+ for bacteria and MEA+ for fungi.
- Contact plates: Contact plates were aged prior to initiation of the study; TSA plates were stored at 30°C to 35°C for 3 days and MEA plates were stored at 26°C to 30°C for 5 days. Using a sterile micropipette, two (2) 50 mcL aliquots of the 1000 CfU/mL standardized culture of a test species were inoculated to the agar surface of the contact plate. Immediately after inoculating, the inoculum was aseptically spread around the entire agar surface. The lid was replaced, and the process was repeated for the other dilutions.
- Envirotest: EnviroTest paddles were aged prior to initiation of the study; TSA paddles were stored at 30°C to 35°C for 3 days, and MEA paddles were stored at 26°C to 30°C for 5 days. Using a sterile micropipette, 50 mcL of the 1000 CfU/mL standardized culture of a test species was inoculated to one side of the EnviroTest paddle. Immediately after inoculating, the inoculum was aseptically spread around the entire agar surface of that side of the EnviroTest paddle. 50 mcL of the 1000 CfU/mL standardized culture was then inoculated to the other side of the paddle and evenly distributed across the surface. The paddle was replaced inside the tube and the screw cap was closed. This process was repeated for the other four dilutions.
- Control plates: Using a sterile micropipette, 2 × 50 mcL of the 1000 CfU/mL standardized culture was delivered to the agar surface, and the inoculum was aseptically spread around the entire surface of the agar. The lid was replaced, and the process was repeated for the other four plates.
- Most probable number scoring and calculation:
- Each plate that had growth was scored as positive (“1”), regardless of the number pf CfU seen on the device.
- Each plate that had no growth was scored as negative (“0”).
- The number of positive plates was totaled for each dilution.
- When calculating MPN values, the intent is to find a 3-dilution sequence to match to an MPN chart. If the lowest dilution resulted all in zeroes, it was discarded. If the highest dilution results all in fives, the next dilution down was used.
- Conversion of growth totals to MPN values were derived from FDA’s BAM Chart.31 MPN values were corrected for the dilution level, understanding that the first 100 mcL delivery is a 10-1 dilution of the original sample.
Results and Discussion
Study #1 Results
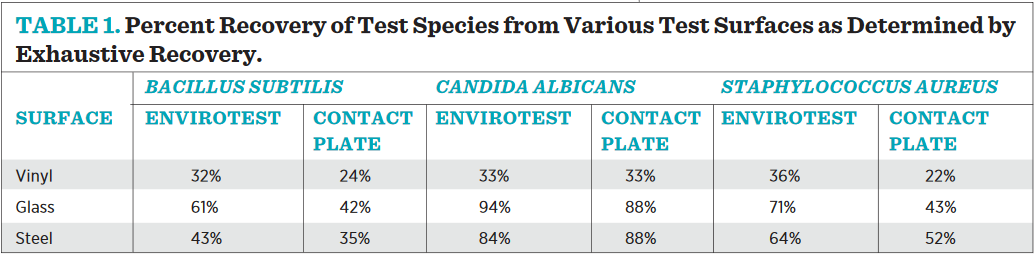
As shown in Table 1, the exhaustive recovery data clearly demonstrate that the contact plates and EnviroTest paddles are comparative methods for determining microbial populations on surfaces, and only a fraction of the population is actually recovered from any surface using either device on the first pressing. This is an important point to remember when setting Alert and Action Levels for an environmental monitoring program, as the counts achieved are meant to be relative to one another and then trended over time; they do not represent an absolute value of microbes on a surface. The exhaustive recovery efficiency is similar on both devices across material types, with vinyl flooring showing the worse initial recovery, and the smooth surfaces of glass and steel offering a better opportunity for high recovery.
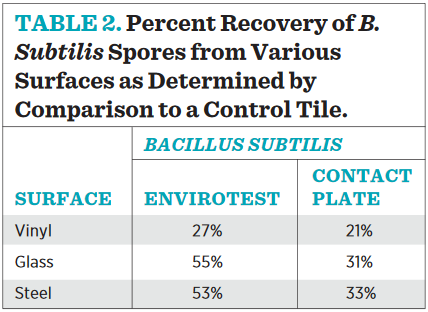
Table 2 demonstrates that Bacillus spores are the ideal test subjects to compare counts between products. They are ubiquitous, airborne, and desiccant resistant, alleviating concern of test species viability during drying cycles. In fact, comparative counts utilizing plate count methods were attempted with vegetative cells, but the results were too variable to make any conclusions. We believe the variability was due to the impact of drying on vegetative culture viability between sampling.
Regardless, when counts of a dried Bacillus inoculum recovered from a surface monitoring device are compared to those recovered by vigorous washing and rinsing, it is clear, once again, that not all surface contaminants are picked up by the agar press on the first pass by either device. The two devices are equivalent in their ability to recover dried spores from compounding surfaces. Repeating the trend witnessed in the exhaustive recovery trial, recovery efficacy in general is better from steel and glass surfaces than from vinyl tile, again underscoring the importance of frequent monitoring and trending similar over time since actual recovery is a relative and not absolute number.
Study #2 Results
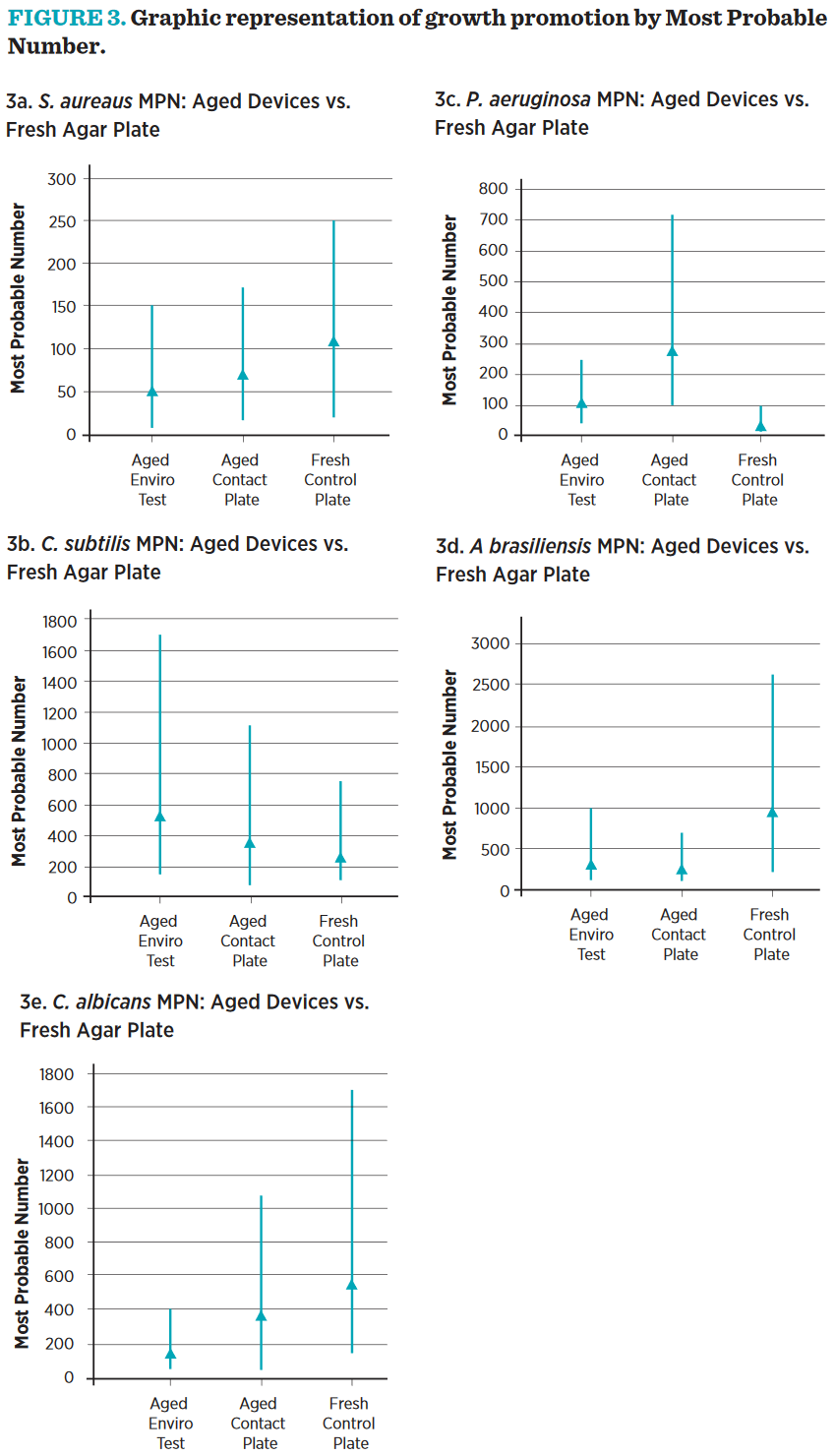
The growth of microorganisms in the different dilutions from identical inocula was scored as “+ growth” or “growth” under the different conditions. These growth patterns were then used, in conjunction with the FDA’s Most Probable Number tables29 to determine the most probable number of cells producing those results, and the 95% confidence intervals around those numbers. The treatments were considered equivalent if the 95%-confidence intervals overlapped, showing each treatment capable of supporting equivalent growth. All treatment groups were equivalent by this measure for all challenge organisms (see Figure 3).
Growth Promotion Discussion
As shown in Figure 2, a CFU is not always a discreet unit and may not represent a single microbial cell or spore. Clumping microbes, fungal ultrastructures, and microbes caught in reproduction would all present a single CFU, even though they are in fact several cells. The ability to suspend dilute quantities of single microbes in small volumes makes low-level inoculation an inherently variable exercise. MPN analysis allows for a statistical calculation of highand lowconfidence interval for each count and offers a good alternative to standard plate count methods by giving confidence intervals in the results.29
In our growth promotion studies, EnviroTest and contact plates MPN confidence levels overlapped each other and a fresh nutrient agar plate. While the absolute number may seem trivially higher in one or the other sampling device, these differences are not statistically significant. The aged EnviroTest, Contact Plates, and fresh control plates all show the same growth promotion ability.
Conclusion
The data generated from these studies verify that the EnviroTest paddle is as effective as conventional contact plates as an environmental monitoring tool (see Table 3). The flat paddle device is widely used in the compounding pharmacy for environmental sampling of surfaces, according to USP <797>, and offers some distinct advantages in terms of shelf-life stability and sampling “ease of use” in comparison to round devices containing the same microbial growth media. Laboratory studies demonstrated equivalence in growth promotion and sampling efficiency between traditional round “contact plates” and the “flat paddle” samplers. Either device is suitable for environmental sampling and compliant with current regulatory requirements.
References
- Cundell AM, Bean R, Massimore L et al. Statistical analysis of environmental monitoring data: Does a worst case time for monitoring clean rooms exist? PDA J Pharm Sci Technol 1998; 52(6): 326–330.
- Hussong D, Madsen RE. Analysis of environmental microbiology data from cleanroom samples. Pharmaceutical Technology 2004; Aseptic Processing: 10–15.
- Farrington JK. Environmental monitoring in pharmaceutical manufacturing—A product risk issue. Am Pharm Rev 2005; 8(4): 26–30.
- Akers J. The proper role of environmental
- Sutton S. Qualification of an environmental monitoring program. J Val Technol 2010; 16(2): 78–82.
- U.S. Department of Health and Human Services. U.S. Food and Drug Administration. Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice. [FDA Website.] September 2004. Available at: www. fda.gov/downloads/Drugs/.../Guidances/ ucm070342.pdf. Accessed July 12, 2014.
- Sutton S. GMP and compounding pharmacies. Amer Pharm Rev 2013; 16: 48–59. [American Pharmaceutical Review Website.] Available at: www.americanpharmaceuticalreview.com/Featured-Articles/135985-GMP-monitoring in aseptic processing. Am Pharm Rev 2006; 9(4): 24–28.
- Grilli A. Microbiology & compounding pharmacies: What the 483s tell us.
Contract Pharma 2013; 15: 52–57.
- Sutton S. FDA expectations for 503B outsourcing facilities. Pharm Purch Prod 2014; 11(10): 18. [PP&P Website.] Available at: www.pppmag.com/ article/1575/October_2014/FDA_Expectations_for_503B_Outsourcing_ Facilities/. Accessed November 2, 2014.
- United States Pharmacopeial Convention, Inc. United States Pharmacopeial 37–National Formulary 32. Chapter <1116> Microbiological Control
and Monitoring of Aseptic Processing Environments. Rockville, MD: US Pharmacopeial Convention, Inc.; 2013: 931–942.
- U.S. Department of Health and Human Services. U.S. Food and Drug Administration. DRAFT Guidance for Industry—Current Good Manufacturing Practice—Interim Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503B of the Federal Food, Drug, and Cosmetic
Act. [FDA Website.] 2014. Available at: www.fda.gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/Guidances/UCM377050.pdf. Accessed July 12, 2014.
- U.S. Department of Health and Human Services. U.S. Food and Drug Administration. DRAFT Guidance—Pharmacy Compounding of Human Drug Products Under Section Current Good Manufacturing
Practice—Interim Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503A of the Federal Food, Drug, and Cosmetic Act. [FDA Website.] 2014. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM377052.pdf. Accessed July 12, 2014.
- United States Pharmacopeial Convention, Inc. United States Pharmacopeial 37–National Formulary 32. Chapter <797> Pharmaceutical Compounding— Sterile Preparations. Rockville, MD: US Pharmacopeial Convention, Inc.; 2013: 410–453.
- Walter WG. Symposium on methods for determining bacterial contamination on surfaces. Bacteriol Rev 1955; 19(4): 284–287.
- Restaino L, Hemphill JB, Frampton EW et al. HYcheck slides versus Rodac plates compared to the swab technique for the recovery of bacteria from hard smooth surfaces. Dairy, Food and Environmental Sanitarians 1994; 14(9): 529–531.
- Andrews W. Determination of microbial load on stainless steel surfaces using Hygicult TPC dipslide. J of AOAC Intl 2003; 86(1): 154–159.
- Hakalehto E. Semmelweis’ present day follow-up: Updating bacterial sampling and enrichment in clinical hygiene. Pathophysiology 2006; 13(4): 257–267.
- PDA. Technical Report #13 (Revised): Fundamentals of an Environmental Monitoring Program. 2014.
- [No author listed.] Environmental monitoring. Pharm Purch Prod
2013; 10(4 State of Pharm Compounding): s30–s31.
- [No author listed.] Environmental monitoring. Pharm Purch Prod
2014; 11(4 State of Pharm Compounding): s28–s29.
- Weissfeld A, Vance P. Understanding the microbiology behind USP <797> Environmental Monitoring. Pharm Purch & Prod 2008; 3(7 Suppl Cleanrooms & Compounding): 2–4.
- Salo S, Laine A, Alanko T et al. Validation of the microbiological methods Hygicult dipslide, contact plate, and swabbing in surface hygiene control: A Nordic collaborative study. J AOAC Int 2000; 83(6): 1357– 1366.
- Salo S, Alanko T, Sjöberq AM et al. Validation of the Hygicult E dipslides method in surface hygiene control: A Nordic collaborative study. J AOAC Int 2002; 85(2): 388–394.
- United States Pharmacopeial Convention, Inc. United States Pharmacopeial 37–National Formulary 32. Chapter <1072> Disinfectants and Antiseptics. Rockville, MD: US Pharmacopeial Convention, Inc.; 2013: 781–785.
- United States Pharmacopeial Convention, Inc. United States Pharmacopeial 37–National Formulary 32. Chapter <71> Sterility Tests. Rockville, MD: US Pharmacopeial Convention, Inc.; 2013: 71–77.
- United States Pharmacopeial Convention, Inc. United States Pharmacopeial 37–National Formulary 32. Chapter <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. Rockville, MD: US Pharmacopeial Convention, Inc.; 2013: 57–62.
- Weenk GH. Microbiological assessment of culture media: Comparison and statistical evaluation of methods. Int J Food Microbiol 1992; 17(2): 159–181.
- U.S. Department of Health and Human Services. U.S. Food and Drug Administration. BAM Media M182: Malt Extract Agar (Yeasts and Molds) (MEAYM). Bacteriological Analytical Manual. [FDA Website.] January 2001. Available at: www.fda.gov/Food/ FoodScienceResearch/LaboratoryMethods/ucm063911.htm. Accessed July 14, 2014.
- U.S. Department of Health and Human Services. U.S. Food and Drug Administration. BAM Media M152: Trypticase (Tryptic) Soy Agar. Bacteriological Analytical Manual. [FDA Website.] January 2001. Available at: www.fda.gov/Food/FoodScienceResearch/ LaboratoryMethods/ucm063779.htm. Accessed July 14, 2014.
- Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev 1994; 58(4): 755–805.
- U.S. Department of Health and Human Services. U.S. Food and Drug Administration. BAM Appendix 2 Most Probable Number from Serial Dilutions. Bacteriological Analytical Manual. [FDA Website.] 2010. Available at: www.fda.gov/Food/FoodScienceResearch/ LaboratoryMethods/ucm109656.htm. Accessed July 12, 2014.
Address correspondence to Scott Sutton, PhD, Principal, Microbiology Network, Inc. E-mail: scott.sutton@microbiologynetwork.com